Characterization and prediction of drug properties using PK-PD modeling
- Predict human PK-PD based on cell and animal study results using modeling and simulation
- Understand drug behavior through PK-PD modeling from the discovery or preclinical stages to inform development strategies
- Design early-phase clinical trials, such as First-In-Human (FIH) studies

Service
- Consulting on pharmacokinetic-pharmacodynamic (PK-PD) data analysis and development strategy in preclinical and clinical stages.
- Preparation of PK-PD data analysis reports.
- Population PK-PD modeling analysis using NONMEM.
- Systems pharmacology and physiologically based pharmacokinetic (PBPK) modeling.
- Development strategy through clinical trial simulation.
- IVIVC(In vitro, in vivo Correlation).
- First-in-human clinical trial design.
- Development of user-friendly tools for on-site application of modeling results.
- Preparation of result reports based on reproducible research.
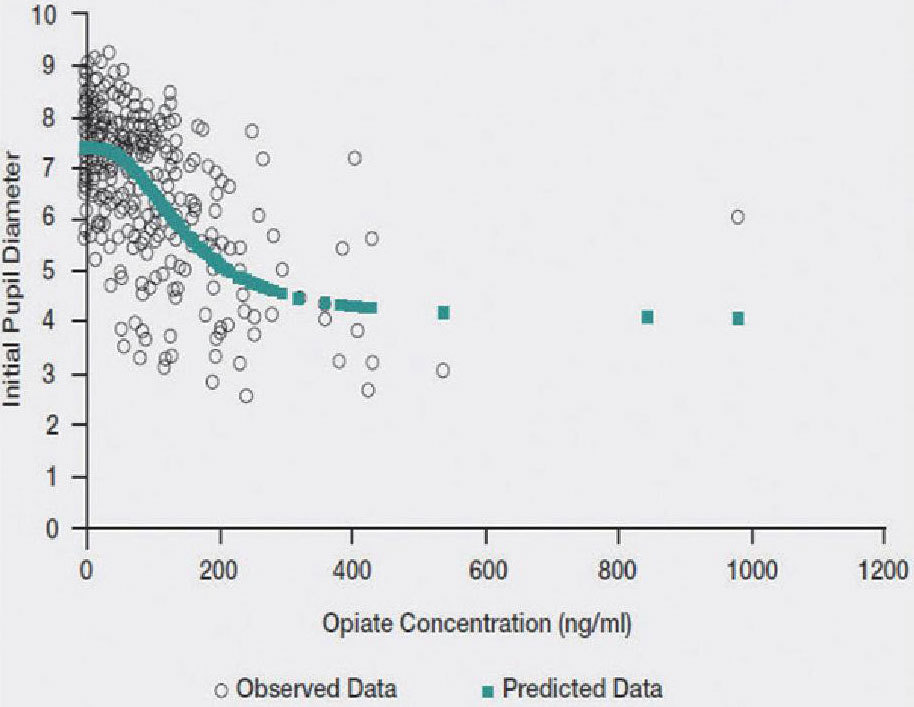
PK-PD Simulation





