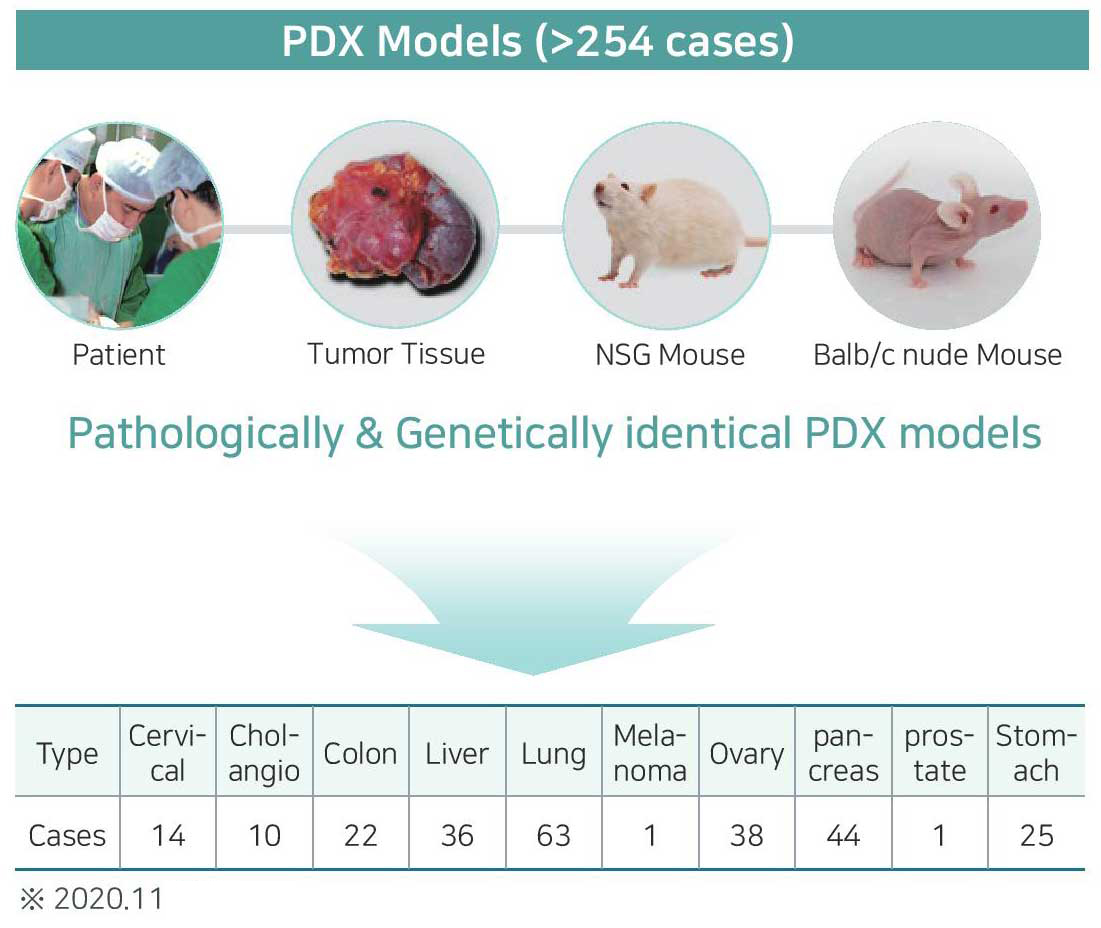
List of Models
|
Patient-Derived primary cells (PDC) |
Panels : liver cancer, ovarian cancer, colorectal cancer, gastric cancer, prostate cancer, lung cancer Clinically annotated patient-derived model Patient-derived models with patient information and whole exome sequencing |
|
Patient-derived xenografts(PDX) |
Panels : colorectal cancer, liver cancer, lung cancer, pancreatic cancer, gastric cancer, renal cell carcinoma, ovarian cancer, uterine cancer, bile duct cancer, prostate cancer etc. |
|
Patient-derived organoid (PDO) |
Panels: Colorectal Cancer, Liver Cancer, Lung Cancer, Gastric Cancer, and normal colon/liver/lung/gastric models. Patient-derived models with available clinical data and mutation profiling, including K-ras, EGFR, Her2 mutations, and Homologous Recombination Deficiency (HRD) diagnostics.
Patient-derived models with clinical data and results from whole exome sequencing and RNA sequencing. |
|
Patient specimens |
Clinically annotated samples: frozen tumor tissues, matched normal tissues, and blood Available in both paraffin-embedded and frozen formats |
Service
- Customized model selection and study design
- Biomarker validation
- Mechanism of action (MOA) and proof of concept (POC) studies
- Efficacy evaluation based on genetic characteristics
- In vitro drug efficacy testing
- Cell viability, cell death assays, and synergy index analysis
- Signal pathway analysis: Immunocytochemistry (ICC), Western blotting, immunoprecipitation, ELISA, quantitative PCR (qPCR)
- Development of patient derived xenograft (PDX) models using organoid:
Simultaneous efficacy evaluation in vitro and in vivo models - Tumor growth inhibition (TGI) and tumor growth delay (TGD) studies
- Kaplan-Meier survival analysis
- Radiotherapy response evaluation
- Histological analysis
- Tissue preparation, immunohistochemistry (IHC), TUNEL assay, and DNA/RNA/protein extraction
Service example
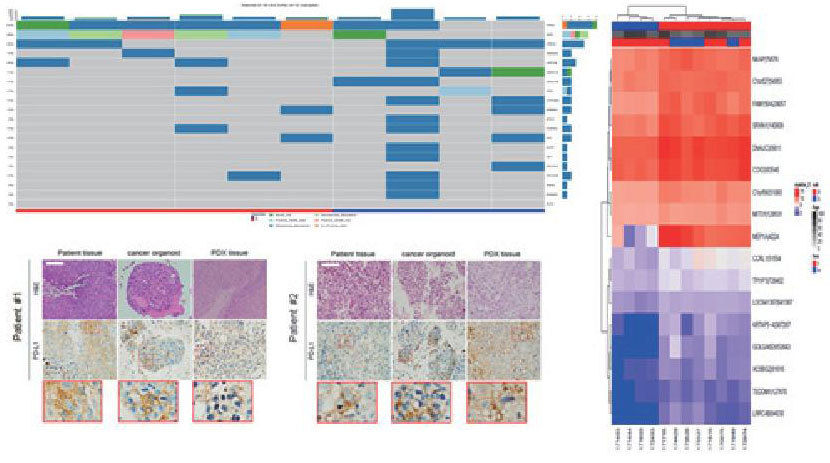
Utilizing patient-derived models
tailored to drug characteristics
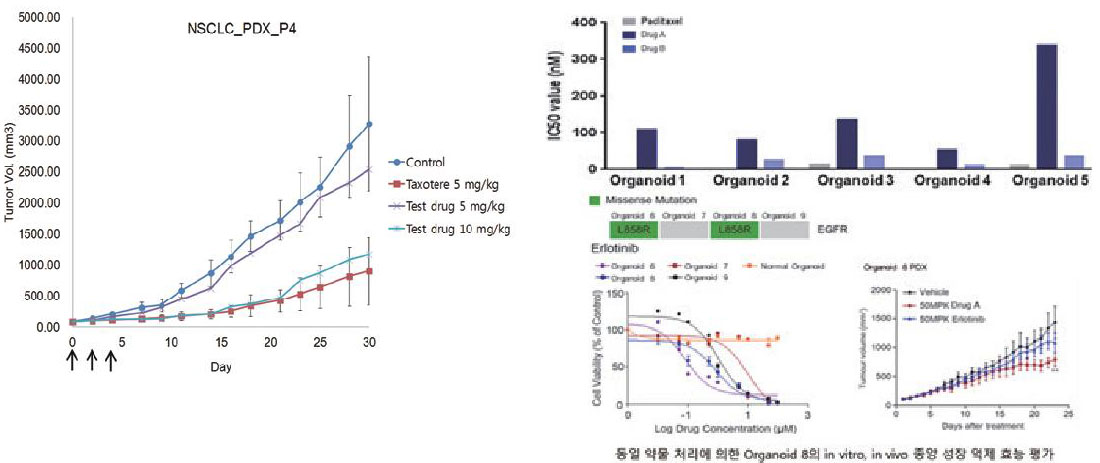
Evaluation of tumor growth inhibition efficacy by drug treatment





