List of Models
|
Cancer type |
Cell line |
Drug |
|
Colon |
COLO205 |
Selumetinib |
|
Leukemia |
MOLM-13 |
Azacitidine, Cytarabine,Decitabine, Quizartinib, FLT3-ITD |
|
THP-1 |
Decitabine |
|
|
K562 |
Daunomycin |
|
|
Lung |
A549 |
AUY922, Cisplatin, Gefitinib,Gemcitabine, Irradiation, Paclitaxel |
|
H209 |
Cisplatin, Etoposide |
|
|
H460 |
Cisplatin, Irradiation, Paclitaxe |
|
|
H1944 |
AUY922 |
|
|
H1975 |
AZD9291, WZ4002 |
|
|
H2228 |
Ceritinib/Lorlatinib, Crizotinib, TAE684, 17-DMAG |
|
|
H3122 |
Ceritinib/Lorlatinib, Crizotinib, 17-DMAG |
|
|
HCC827 |
Cisplatin, Erlotinib, Gefitinib, Paclitaxel, WZ4002 |
|
|
PC-9 |
AZD9291, Cisplatin, Erlotinib, Erlotinib/WZ4002, Gefitinib, Gefitinib/ WZ4002, Paclitaxel, WZ4002 |
|
|
Ovary |
A2780-CP |
Cisplatin |
|
NCI/ADR-RES |
Multidrug |
|
|
Other |
Ba/F3/AXL Ba/F3/del19 EGFR Ba/F3/del19+T790M+C797S Ba/F3/L858R Ba/F3/L858R+T790M+C797S |
Ba/F3/CCDC6-RET Ba/F3/del19+T790M Ba/F3/EML4-ALK Ba/F3/L858R+T790M Ba/F3/FLT3-ITD |
Service
-
Studies for novel anti-cancer drug in resistance cell line panels
- Evaluation of overcoming resistance
- Comparison with open-label global new drugs through screening
- Cell viability / Death assay / IC50 calculation
- In vivo TGI effects, Ex vivo screening
- Evaluation of resistance-specific biomarkers
- Pre-validated resistance candidate gene library
- MOA & POC validation
- Effective combination strategy
- Cell viability between a novel drug and resistance candidate gene target drug
- Synergy index
- Validation of synergistic target gene
Service example
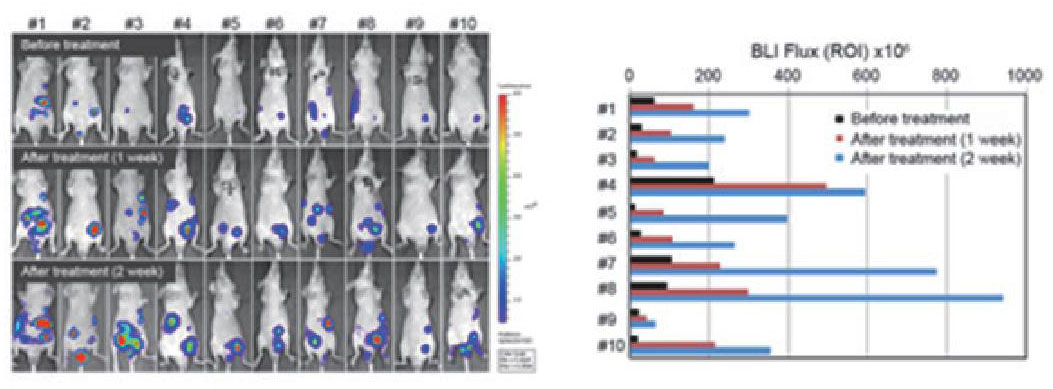
Evaluation of resistance overcoming
in vivo studies using orthotopic xenograft model
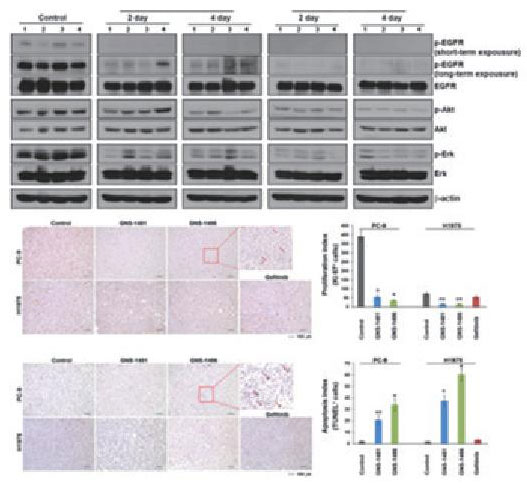
Validation of drug efficacy by molecular analysis





